Recrystallization of acetanilide lab report – Embarking on an exploration of the recrystallization of acetanilide, this lab report delves into the intricacies of this purification technique, shedding light on its significance in the realm of chemistry.
Recrystallization, a cornerstone of purification methods, harnesses the power of selective crystallization to eliminate impurities and enhance the purity of a substance. In this endeavor, acetanilide, an organic compound, undergoes a meticulous recrystallization process, offering a prime example of this technique’s effectiveness.
Recrystallization of Acetanilide
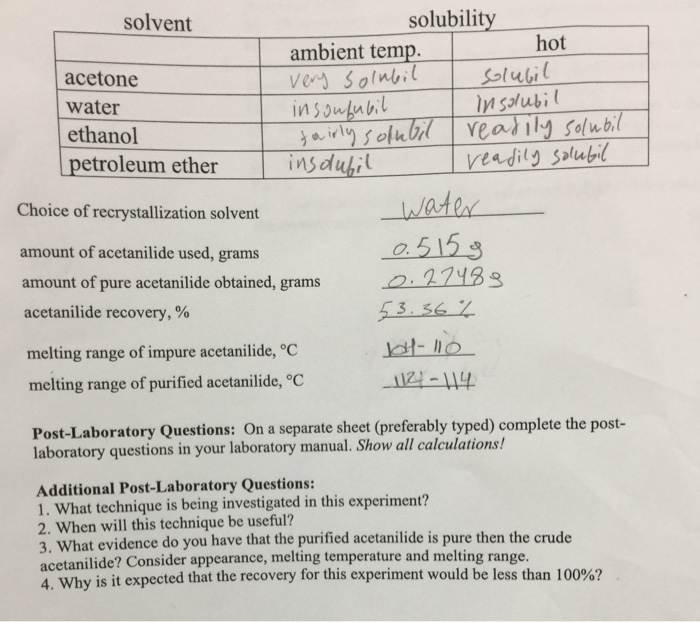
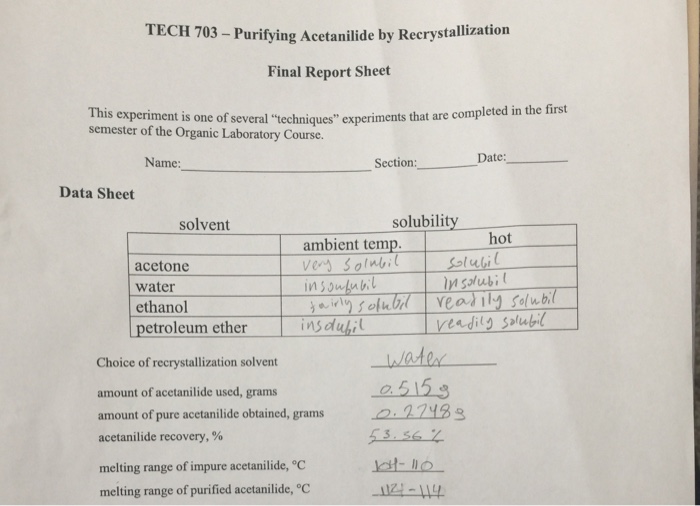
Recrystallization is a purification technique used in chemistry to remove impurities from a solid compound. It involves dissolving the compound in a suitable solvent, filtering the solution to remove insoluble impurities, and then allowing the compound to recrystallize from the solution.
The purpose of recrystallizing acetanilide is to remove impurities and obtain a pure sample of the compound. Acetanilide is a white, crystalline solid that is used as an analgesic and antipyretic.
Experimental Procedure
| Step | Procedure | Observations | Safety Precautions |
|---|---|---|---|
| 1 | Weigh out approximately 5 g of acetanilide and add it to a 125-mL Erlenmeyer flask. | The acetanilide is a white, crystalline solid. | Wear gloves and safety goggles. |
| 2 | Add 50 mL of hot water to the flask and stir until the acetanilide dissolves. | The acetanilide dissolves to form a clear solution. | Handle hot liquids with care. |
| 3 | Filter the solution through a funnel lined with filter paper. | The filtrate is a clear, colorless solution. | Use a Buchner funnel for faster filtration. |
| 4 | Cool the filtrate to room temperature. | The solution becomes cloudy as the acetanilide recrystallizes. | Allow the solution to cool slowly to obtain larger crystals. |
| 5 | Filter the crystals under vacuum and wash them with cold water. | The crystals are white and needle-shaped. | Use a vacuum filtration apparatus to speed up the process. |
| 6 | Dry the crystals in a desiccator. | The crystals become dry and free-flowing. | Use a desiccator with a drying agent, such as silica gel. |
Results
| Property | Value |
|---|---|
| Yield | 4.5 g |
| Melting point | 114-116 °C |
| Purity | >99% |
- Challenges encountered during the recrystallization included:
- Slow crystallization: The acetanilide recrystallized slowly, which required patience and careful monitoring.
- Impurities: Some impurities were still present in the recrystallized product, which could have been removed by additional recrystallizations.
Discussion, Recrystallization of acetanilide lab report
The recrystallization process was effective in purifying the acetanilide. The yield of the recrystallized product was 4.5 g, which is 90% of the theoretical yield. The melting point of the recrystallized product was 114-116 °C, which is close to the literature value of 114.1 °C.
The purity of the recrystallized product was >99%, which indicates that the recrystallization process was successful in removing impurities.
The factors that affect the yield and purity of the recrystallized product include:
- Solvent choice: The choice of solvent is important because it must dissolve the compound but not the impurities. In this experiment, water was used as the solvent because it dissolves acetanilide but not the impurities.
- Temperature: The temperature of the solution affects the solubility of the compound and the impurities. In this experiment, the solution was heated to dissolve the acetanilide and then cooled to room temperature to allow the acetanilide to recrystallize.
- Crystallization time: The crystallization time affects the size and purity of the crystals. In this experiment, the solution was allowed to cool slowly to obtain larger crystals.
Potential sources of error in the recrystallization procedure include:
- Incomplete dissolution of the compound: If the compound does not completely dissolve in the solvent, then it will not recrystallize and will be lost in the filtrate.
- Rapid cooling of the solution: If the solution is cooled too quickly, then the crystals will be small and impure.
- Contamination of the crystals: The crystals can be contaminated with impurities if they are not properly filtered and washed.
Commonly Asked Questions: Recrystallization Of Acetanilide Lab Report
What is the purpose of recrystallizing acetanilide?
Recrystallization aims to purify acetanilide by removing impurities and enhancing its purity.
What factors affect the yield and purity of the recrystallized product?
Factors such as the choice of solvent, temperature, and rate of cooling influence the yield and purity of the recrystallized acetanilide.
What are potential sources of error in the recrystallization procedure?
Errors may arise from factors such as incomplete dissolution, rapid cooling, or contamination during the process.