Devise a 3-step synthesis of 1-propanol from 2-propanol – In the realm of organic chemistry, the synthesis of 1-propanol from 2-propanol stands as a cornerstone reaction. This three-step process involves a series of intricate transformations, each governed by distinct mechanisms and reagents. Embark on this journey as we delve into the intricacies of this fascinating synthesis, exploring the oxidation, hydration, and reduction reactions that culminate in the formation of 1-propanol.
The oxidation of 2-propanol to acetone marks the initial step of this synthetic endeavor. This reaction entails the transfer of electrons from the alcohol to an oxidizing agent, resulting in the formation of a carbonyl group. Subsequently, the hydration of acetone to isopropanol introduces a hydroxyl group to the molecule, paving the way for the final reduction step.
In this concluding stage, isopropanol undergoes reduction to yield the target product, 1-propanol. Throughout this synthetic odyssey, we will encounter a diverse array of reagents and catalysts, each playing a pivotal role in facilitating these transformations.
Synthesis of 1-propanol from 2-propanol
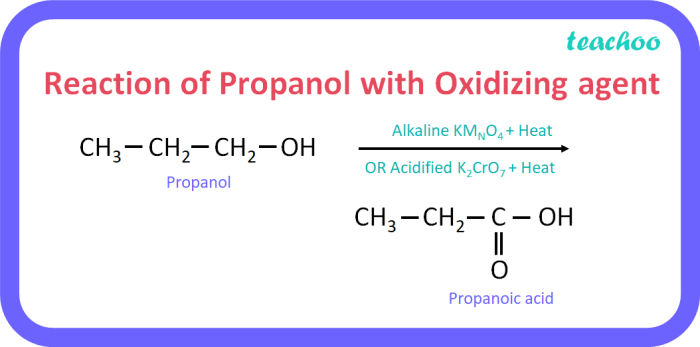
1-propanol is a primary alcohol that can be synthesized from 2-propanol through a three-step process involving oxidation, hydration, and reduction. This synthesis is of great importance in the chemical industry as 1-propanol is widely used as a solvent, in the production of pharmaceuticals, and as a fuel additive.
Oxidation of 2-propanol to acetone
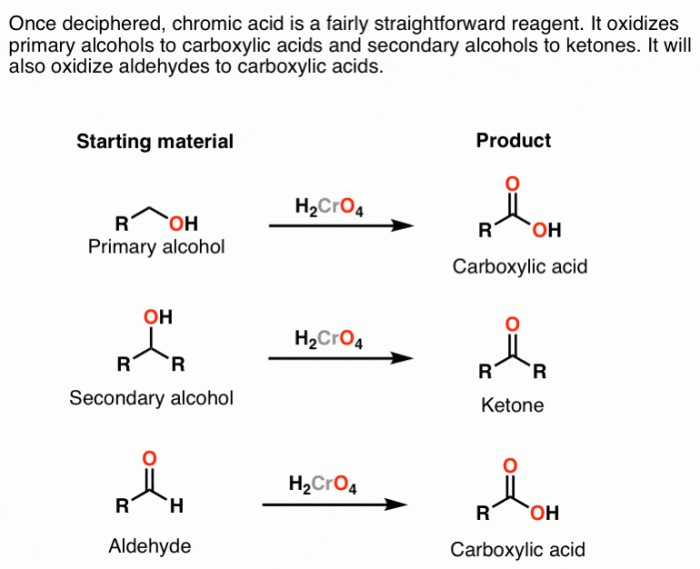
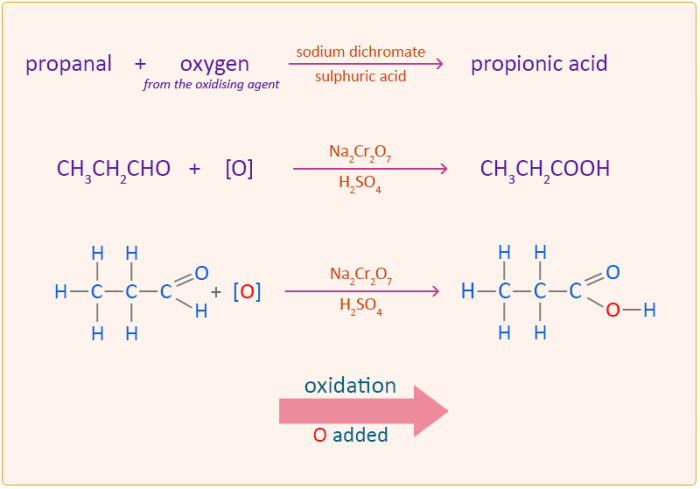
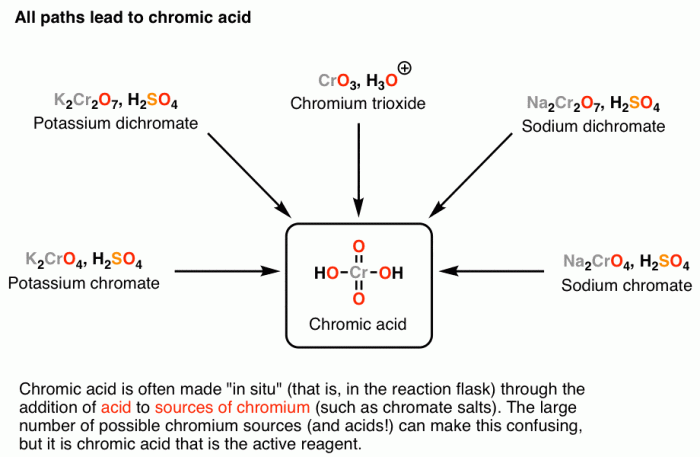
The first step in the synthesis is the oxidation of 2-propanol to acetone. This reaction is typically carried out using an oxidizing agent such as potassium permanganate (KMnO4) or chromic acid (H2CrO4) in an acidic medium.
The mechanism of the oxidation reaction involves the transfer of two electrons from the alcohol to the oxidizing agent, resulting in the formation of a ketone (acetone) and water.
The reagents and conditions required for the oxidation reaction are as follows:
- 2-propanol
- Oxidizing agent (e.g., KMnO4 or H2CrO4)
- Acidic medium (e.g., H2SO4 or HCl)
Hydration of acetone to isopropanol
The second step in the synthesis is the hydration of acetone to isopropanol. This reaction is typically carried out in the presence of a catalyst such as sulfuric acid (H2SO4) or hydrochloric acid (HCl).
The mechanism of the hydration reaction involves the addition of water to the carbonyl group of acetone, resulting in the formation of a gem-diol intermediate. This intermediate then undergoes a proton transfer to form isopropanol.
The catalyst plays a crucial role in the reaction by providing a proton for the proton transfer step.
The reagents and conditions required for the hydration reaction are as follows:
- Acetone
- Catalyst (e.g., H2SO4 or HCl)
- Water
Reduction of isopropanol to 1-propanol, Devise a 3-step synthesis of 1-propanol from 2-propanol
The final step in the synthesis is the reduction of isopropanol to 1-propanol. This reaction is typically carried out using a reducing agent such as sodium borohydride (NaBH4) or lithium aluminum hydride (LiAlH4).
The mechanism of the reduction reaction involves the transfer of two electrons from the reducing agent to the alcohol, resulting in the formation of a primary alcohol (1-propanol) and a metal salt.
The reagents and conditions required for the reduction reaction are as follows:
- Isopropanol
- Reducing agent (e.g., NaBH4 or LiAlH4)
- Protic solvent (e.g., methanol or ethanol)
Frequently Asked Questions: Devise A 3-step Synthesis Of 1-propanol From 2-propanol
What is the primary oxidizing agent used in the oxidation step?
Potassium permanganate (KMnO4) is commonly employed as the oxidizing agent in this step.
What is the role of sulfuric acid in the hydration step?
Sulfuric acid acts as a catalyst, promoting the addition of water to the carbonyl group.
Which reducing agent is typically used in the reduction step?
Sodium borohydride (NaBH4) is a commonly used reducing agent for this step.